вторник, 30 октября 2018 г.
воскресенье, 30 сентября 2018 г.
Science of Heat
Science of Heat
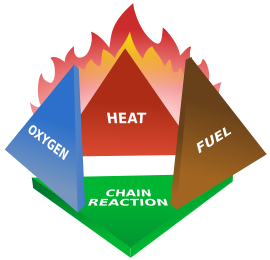
Heat is the transfer of energy from a one object to another due to a difference in temperature. Heat can be measured in joules, BTUs (British thermal unit), or calories.
Heat and temperature are closely related, but they are not the same thing. The temperature of an object is determined by how fast its molecules are moving. The faster the molecules are moving the higher the temperature. We say objects that have a high temperature are hot and objects with a low temperature are cold.
Transferring of Heat
When two items are combined or touching each other, their molecules will transfer energy called heat. They will try to come to a point where they both have the same temperature. This is called equilibrium. Heat will flow from the hotter object to the colder. The molecules in the hotter object will slow down and the molecules in the colder object will speed up. Eventually they will get to the point where they have the same temperature.
This happens all the time around you. For example, when you take an ice cube and put it into a warm soda. The ice cube will become warmer and melt, while the soda will cool down.
Hot Objects Expand
When something gets hotter it will expand, or get bigger. At the same time, when something gets colder it will shrink. This property is used to make mercury thermometers. The line in the thermometer is actually liquid mercury. As the liquid gets hotter, it will expand and rise in the thermometer to show a higher temperature. It's the expansion and contraction due to temperature that allows the thermometer to work.
Heat Conduction
When heat transfers from one object to another, this is called conduction. Some materials conduct heat better than others. Metal, for example, is a good conductor of heat. We use metal in pots and pans to cook because it will move the heat from the flame to our food quickly. Cloth, like a blanket, isn't a good conductor of heat. Because it's not a good conductor, a blanket works well to keep us warm at night as it won't conduct the heat from our bodies out to the cold air.
Matter Changing State
Heat has an impact on the state of matter. Matter can change state based on heat or temperature. There are three states that matter can take depending on its temperature: solid, liquid, and gas. For example, if water is cold and its molecules are moving very slow, it will be a solid (ice). If it warms up some, the ice will melt and water becomes a liquid. If you add a lot of heat to water, the molecules will move very fast and it will become a gas (steam).
Heat and temperature are closely related, but they are not the same thing. The temperature of an object is determined by how fast its molecules are moving. The faster the molecules are moving the higher the temperature. We say objects that have a high temperature are hot and objects with a low temperature are cold.
Transferring of Heat
When two items are combined or touching each other, their molecules will transfer energy called heat. They will try to come to a point where they both have the same temperature. This is called equilibrium. Heat will flow from the hotter object to the colder. The molecules in the hotter object will slow down and the molecules in the colder object will speed up. Eventually they will get to the point where they have the same temperature.
This happens all the time around you. For example, when you take an ice cube and put it into a warm soda. The ice cube will become warmer and melt, while the soda will cool down.
Hot Objects Expand
When something gets hotter it will expand, or get bigger. At the same time, when something gets colder it will shrink. This property is used to make mercury thermometers. The line in the thermometer is actually liquid mercury. As the liquid gets hotter, it will expand and rise in the thermometer to show a higher temperature. It's the expansion and contraction due to temperature that allows the thermometer to work.
Heat Conduction
When heat transfers from one object to another, this is called conduction. Some materials conduct heat better than others. Metal, for example, is a good conductor of heat. We use metal in pots and pans to cook because it will move the heat from the flame to our food quickly. Cloth, like a blanket, isn't a good conductor of heat. Because it's not a good conductor, a blanket works well to keep us warm at night as it won't conduct the heat from our bodies out to the cold air.
Heat has an impact on the state of matter. Matter can change state based on heat or temperature. There are three states that matter can take depending on its temperature: solid, liquid, and gas. For example, if water is cold and its molecules are moving very slow, it will be a solid (ice). If it warms up some, the ice will melt and water becomes a liquid. If you add a lot of heat to water, the molecules will move very fast and it will become a gas (steam).
понедельник, 28 мая 2018 г.
Comparative adjectives
COMPARATIVE ADJECTIVES
Comparative adjectives are used to compare differences between the two objects they modify (larger, smaller, faster, higher). They are used in sentences where two nouns are compared, in this pattern:
Noun (subject) + verb + comparative adjective + than + noun (object).
The second item of comparison can be omitted if it is clear from the context (final example below).
EXAMPLES
- My house is larger than hers.
- This box is smaller than the one I lost.
- Your dog runs faster than Jim's dog.
- The rock flew higher than the roof.
- Jim and Jack are both my friends, but I like Jack better. ("than Jim" is understood)
SUPERLATIVE ADJECTIVES
Superlative adjectives are used to describe an object which is at the upper or lower limit of a quality (the tallest, the smallest, the fastest, the highest). They are used in sentences where a subject is compared to a group of objects.
Noun (subject) + verb + the + superlative adjective + noun (object).
The group that is being compared with can be omitted if it is clear from the context (final example below).
EXAMPLES
- My house is the largest one in our neighborhood.
- This is the smallest box I've ever seen.
- Your dog ran the fastest of any dog in the race.
- We all threw our rocks at the same time. My rock flew the highest. ("of all the rocks" is understood)
FORMING REGULAR COMPARATIVES AND SUPERLATIVES
Forming comparatives and superlatives is easy. The form depends on the number of syllables in the original adjective.
ONE SYLLABLE ADJECTIVES
Add -er for the comparative and -est for the superlative. If the adjective has a consonant + single vowel + consonant spelling, the final consonant must be doubled before adding the ending.
| Adjective | Comparative | Superlative |
|---|---|---|
| tall | taller | tallest |
| fat | fatter | fattest |
| big | bigger | biggest |
| sad | sadder | saddest |
TWO SYLLABLES
Adjectives with two syllables can form the comparative either by adding -er or by preceeding the adjective with more. These adjectives form the superlative either by adding -est or by preceeding the adjective with most. In many cases, both forms are used, although one usage will be more common than the other. If you are not sure whether a two-syllable adjective can take a comparative or superlative ending, play it safe and use moreand most instead. For adjectives ending in y, change the y to an i before adding the ending.
| Adjective | Comparative | Superlative |
|---|---|---|
| happy | happier | happiest |
| simple | simpler | simplest |
| busy | busier | busiest |
| tilted | more tilted | most tilted |
| tangled | more tangled | most tangled |
THREE OR MORE SYLLABLES
Adjectives with three or more syllables form the comparative by putting more in front of the adjective, and the superlative by putting most in front.
| Adjective | Comparative | Superlative |
|---|---|---|
| important | more important | most important |
| expensive | more expensive | most expensive |
IRREGULAR COMPARATIVES AND SUPERLATIVES
These very common adjectives have completely irregular comparative and superlative forms.
| Adjective | Comparative | Superlative |
|---|---|---|
| good | better | best |
| bad | worse | worst |
| little | less | least |
| much | more | most |
| far | further / farther | furthest / farthest |
воскресенье, 29 апреля 2018 г.
Energy
Energy
What is Energy?The simplest definition of energy is "the ability to do work". Energy is how things change and move. It's everywhere around us and takes all sorts of forms. It takes energy to cook food, to drive to school, and to jump in the air.
Different forms of Energy
Energy can take a number of different forms. Here are some examples:
- Chemical - Chemical energy comes from atoms and molecules and how they interact.
- Electrical - Electrical energy is generated by the movement of electrons.
- Gravitational - Large objects such as the Earth and the Sun create gravity and gravitational energy.
- Heat - Heat energy is also called thermal energy. It comes from molecules of different temperatures interacting.
- Light - Light is called radiant energy. The Earth gets a lot of its energy from the light of the Sun.
- Motion - Anything that is moving has energy. This is also called kinetic energy.
- Nuclear - Huge amounts of nuclear energy can be generated by splitting atoms.
- Potential - Potential energy is energy that is stored. One example of this is a spring that is pressed all the way down. Another example is a book sitting high on a shelf.
In physics, the standard unit of measure for energy is the joule which is abbreviated as J. There are other units of measure for energy that are used throughout the world including kilowatt-hours, calories, newton-meters, therms, and foot-pounds.
Law of Conservation of Energy
This law states that energy is never created or destroyed, it is only changed from one state to another. One example is the chemical energy in food that we turn into kinetic energy when we move.
Renewable and Nonrenewable
As humans we use a lot of energy to drive our cars, heat and cool our houses, watch TV, and more. This energy comes from a variety of places and in a number of forms. Conservationists classify the energy we use into two types: renewable and nonrenewable. Nonrenewable energy uses up resources that we cannot recreate. Some examples of this are gas to run our car and coal burned in power plants. Once they are used, they are gone forever. A renewable energy source is one that can be replenished. Examples of this include hydropower from turbines in a dam, wind power from windmills, and solar power from the sun.
Fun Facts about Energy
- In 2008 about 7% of the energy used in the United States was from renewable sources.
- A modern windmill or turbine can generate enough electricity to power around 300 homes.
- People have used waterpower to grind grain for over 2,000 years.
- Geothermal power uses energy from geysers, hot springs, and volcanoes.
- The entire world could be powered for a year from the energy from the sun that falls on the Earth's surface in one hour. We just need to figure out how to harness it!
Подписаться на:
Сообщения (Atom)

